Science
Autologous Cellular Therapy
Autologous cellular therapy has recently emerged as a credible and practical treatment option for cancer and other highly debilitating diseases.
BrainStorm is focused on developing clinical-stage autologous cellular therapy as a potentially transformative approach to treating neurodegenerative diseases.
BrainStorm has developed a targeted, innovative, proprietary and validated autologous cellular technology platform (NurOwn®) for the treatment of neurodegenerative diseases.
MSC-NTF Cells
Autologous MSC-NTF cells represent a promising therapeutic candidate by targeting disease pathways important in neurodegenerative disorders.
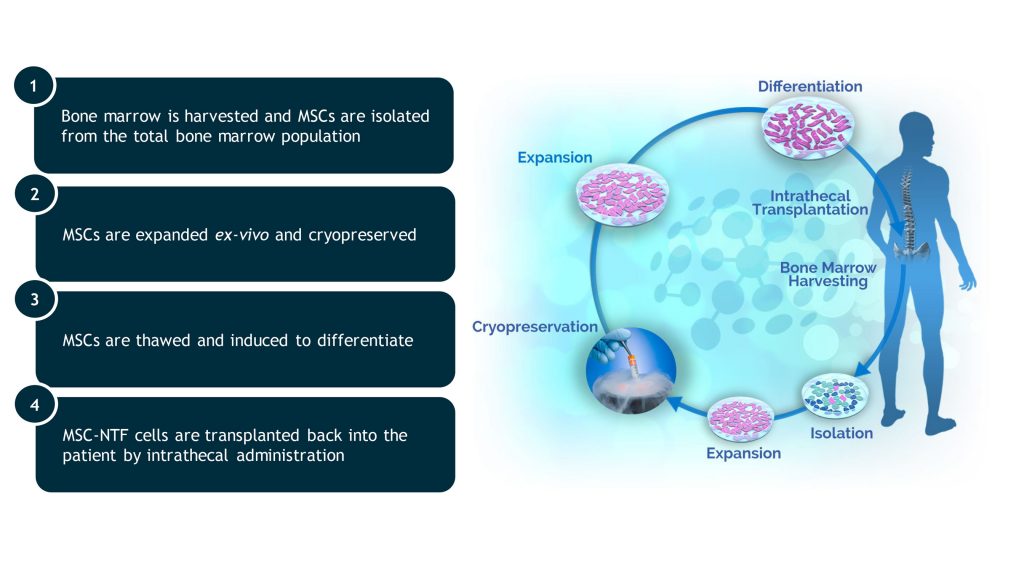
Autologous MSC-NTF cells are produced from the patient’s own bone marrow-derived MSCs that have been differentiated in culture.
A patient’s own MSCs are harvested and differentiated to secrete high levels of NTFs using a proprietary technology. The differentiated MSCs, known as MSC-NTF cells, are then harvested and prepared for injection into the patient. The MSC-NTF cells are not genetically modified.
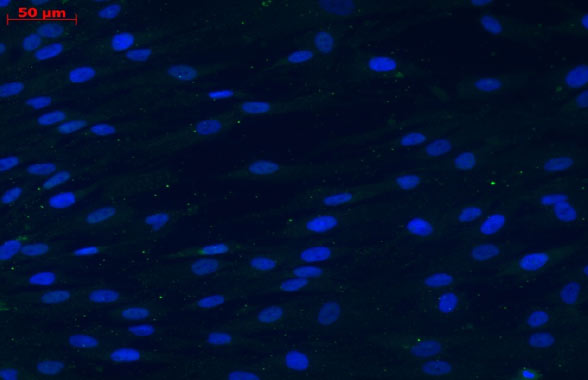
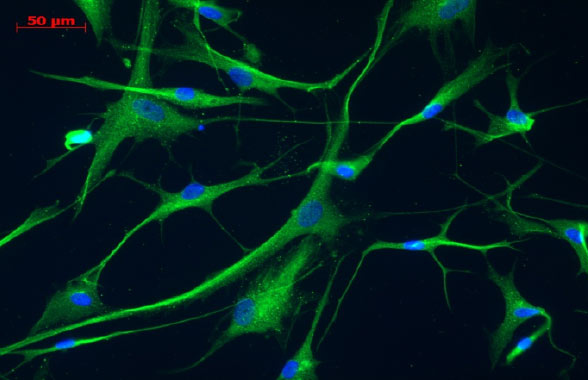
Source: BrainStorm, Data on file. Detection of GDNF expression in MSC and MSC-NTF cells by immunofluorescence.
NTF Levels before and after differentation
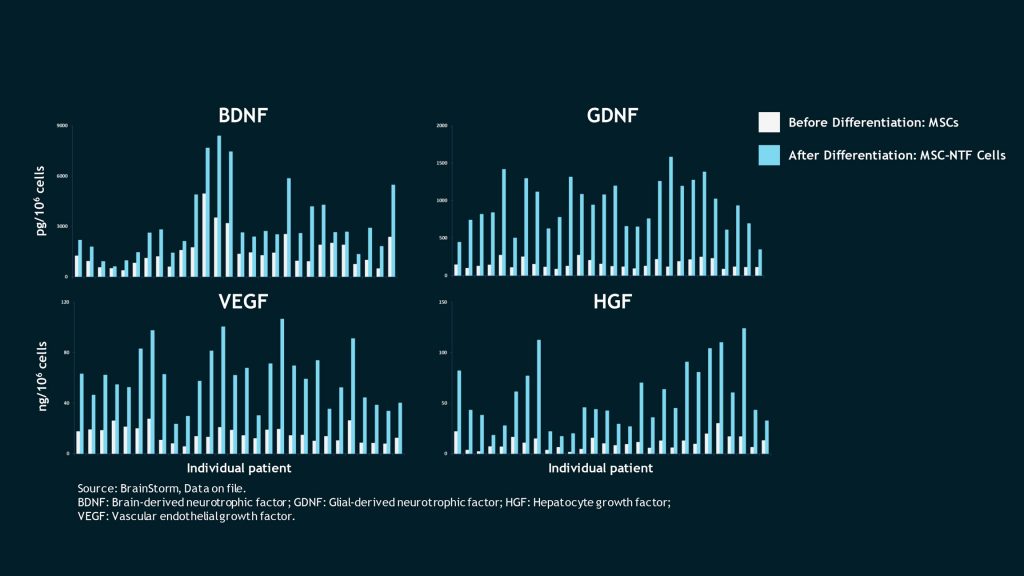
Autologous MSC-NTF cells secrete a unique profile of bioactive molecules, including NTFs, microRNA and cytokines
Following intrathecal administration, the autologous MSC-NTF cells may activate neuroprotective and immunomodulatory pathways
MSC-NTF Cell Production
BrainStorm has pioneered production of autologous MSC-NTF cells. We have developed proprietary methods to engineer, produce, and purify autologous MSC-NTF cells at a scale and quality necessary to bring MSC-NTF therapeutics to patients with debilitating neurodegenerative diseases.
Each treatment consists of a ready-for-injection syringe containing 100-125 x 106 freshly-harvested autologous MSC-NTF cells in a volume of 4 mL. The MSC-NTF cells are autologous and therefore unlikely to induce an adverse immune response.
Dana-Farber Cancer Institute (Dana-Farber) in Boston, Massachusetts and the City of Hope National Medical Center in Duarte, California provided clean room facilities for production of autologous MSC-NTF cells for the Phase 3 Clinical Trial in ALS.
PATENTS
BrainStorm has a robust intellectual property portfolio.
We hold rights to clinical development and commercialization of the NurOwn® technology platform through an exclusive, worldwide licensing agreement.
RECENT BRAINSTORM PUBLICATIONS AND PRESENTATIONS
Debamestrocel multimodal effects on biomarker pathways in amyotrophic lateral sclerosis are linked to clinical outcomes
Erratum issued to A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
Supplemental file with updated graphs from the Erratum – A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis
Therapeutic Benefits of MSC-NTF (NurOwn®) Exosomes in Acute Lung Injury Models
