OUR MISSION
BrainStorm is dedicated to defeating neurodegenerative diseases using an innovative, best-in-class, autologous cellular therapeutic technology platform, NurOwn®.
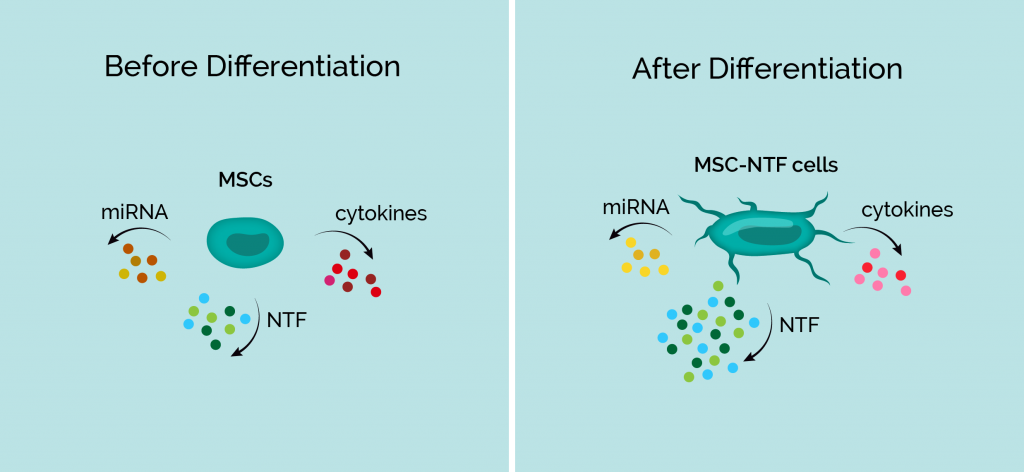
NUROWN® TECHNOLOGY PLATFORM
BrainStorm’s autologous MSC-NTF cell therapy is investigational and not FDA approved.
Defining a new class of autologous cellular therapeutics
Grounded in breakthrough scientific innovation and focused on unmet medical need, BrainStorm is building a best-in-class platform, NurOwn®, for production of commercial-ready MSC-NTF cells to treat highly debilitating neurodegenerative diseases that currently have limited treatment options.
WHAT WE DO
BrainStorm Cell Therapeutics, Inc. (NASDAQ: BCLI) is a clinical-stage, biotechnology company focused on the development of best-in-class autologous cellular therapeutics for the treatment of highly debilitating neurodegenerative diseases.