Pipeline
PIPELINE
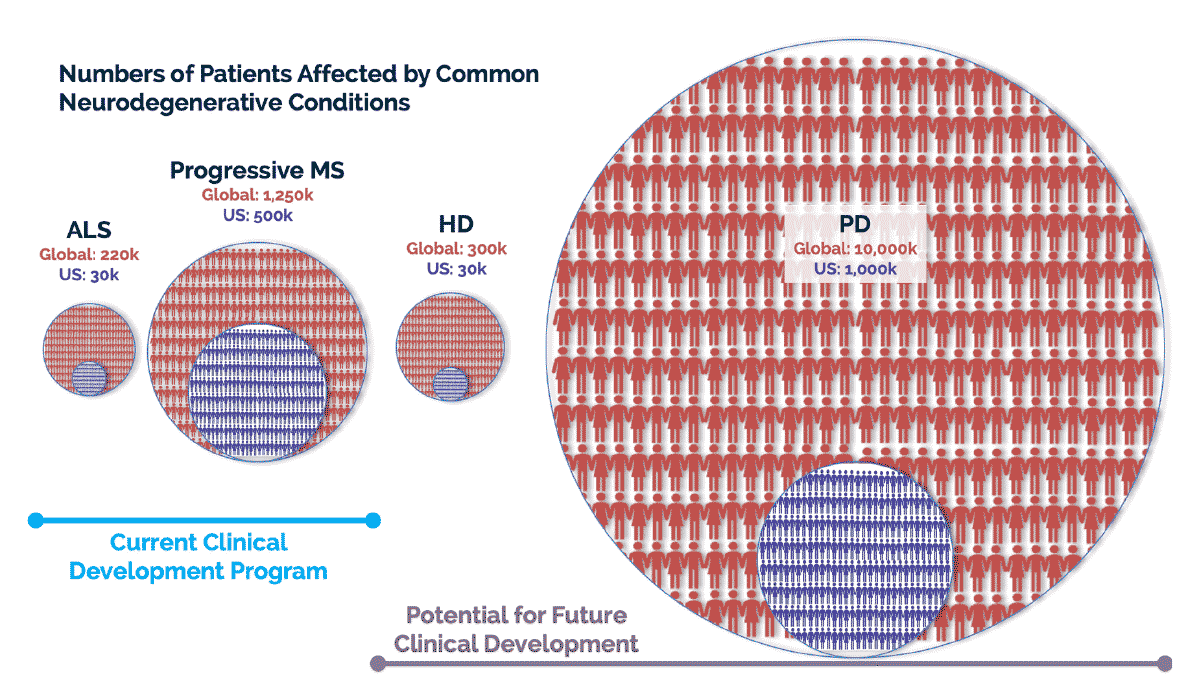
There is significant unmet need for effective therapies for those people whose lives are disrupted by highly debilitating and often fatal neurodegenerative diseases.
Using the NurOwn® technology platform, BrainStorm is focused on realizing the potential of life-changing autologous cellular therapies for the treatment of debilitating neurodegenerative diseases.
Clinical Development Program for Autologous MSC-NTF Cellular TherapY
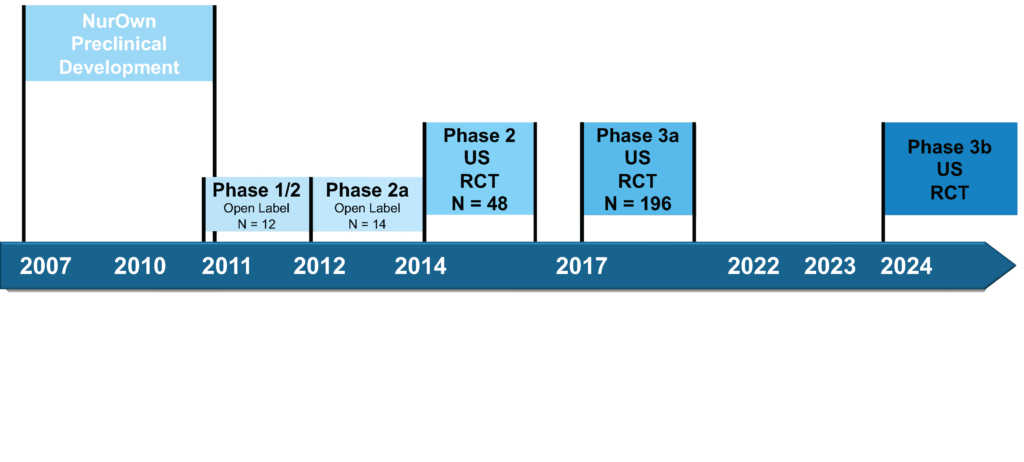
We anticipate commencement of a Phase 3b trial in ALS in 2024. A Phase 3a clinical trial in autologous MSC-NTF cellular therapy in Amyotrophic Lateral Sclerosis (ALS) and a Phase 2 clinical trial in Progressive MS was completed.
We are conducting preclinical studies to determine the potential for autologous MSC-NTF cells in other conditions, including Huntington’s Disease (HD), Parkinson’s Disease (PD) and Autism Spectrum Disorder.
About ALS
ALS is a relentlessly progressive neurodegenerative disease characterized by selective deterioration of cortical, brainstem, and spinal cord motor neurons. As motor neurons die, patients become weaker and lose motor function. For most patients, the disease leads to death within 3 to 5 years of diagnosis. Currently available treatment does not halt or reverse disease progression in ALS.1
Clinical Development Program for Autologous MSC-NTF Cells in ALS
Summary of Clinical development program of BrainStorm’s Autologous MSC-NTF Cellular Therapy in ALS
Autologous MSC-NTF cells have been administered to approximately 170 ALS patients in four completed clinical trials, including two open-label trials in Israel and two multicenter, double-blind, placebo-controlled trials in the United States. Overall, the results show administration of autologous MSC-NTF cells is safe and well-tolerated.
Study | N | Outcomes |
|---|---|---|
Phase 1 / 2 (Israel) Single-arm, single dose NCT01051882 | 12 | Administration of autologous MSC-NTFs was found to be safe and well-tolerated during these 6-month trials |
Phase 2a (Israel) Single-arm, single ascending dose NCT01777646 | 14 | Clinically meaningful improvements in the rate of disease progression for the six months following treatment were observed on both the ALSFRS-R and FVC.
See publication in JAMA Neurology |
Phase 2 (United States) Randomized, placebo-controlled, double-blind, single dose NCT02017912 | 48 | Administration of autologous MSC-NTF cells was found to be safe and well tolerated with the majority of adverse events being mild or moderate in severity
Reduction in rate of ALS disease progression was observed in the autologous MSC-NTF treatment group but not in the placebo treatment group, and the treatment effect was statistically significant in a subpopulation of participants with more rapid disease progression.
CSF biomarkers confirmed the secretion of NTFs and a reduction in inflammatory activity in the patients treated MSC-NTF-treated. See publication in Neurology |
Phase 3a (United States) Randomized, placebo-controlled, double-blind, multi-dose NCT03280056 | 196 | Administration of MSC-NTF treatment was well tolerated; there were no safety concerns. The primary endpoint was not met, however a pre-specified analysis of participants with baseline ALSFRS-R ≥ 35 (n = 58) showed a clinical response rate at 28 wk. Significant improvements in cerebrospinal biomarkers of neuroinflammation, neurodegeneration, and neurotrophic factor support were observed with MSC-NTF, with placebo unchanged. The study pre-specified subgroup suggests that MSC-NTF participants with less severe disease may have retained more function compared to placebo. See publication in Muscle & Nerve |
About Planned ALS phase 3b trial and Completed ALS phase 3a trial (NCT03280056)
BrainStorm completed a Phase 3a trial using repeat-administration of autologous MSC-NTF cells in ALS at six leading clinical sites in the United States, supported by a grant from the California Institute for Regenerative Medicine (CIRM CLIN2-0989). The multi-center Phase 3 study enrolled 189 patients with ALS randomized 1:1 that received autologous MSC-NTF cells or placebo and evaluated ALS Functional Rating Scale–Revised (ALSFRS-R) responder analysis as a primary efficacy outcome measure (NCT03280056). A diverse study population made over all interpretation difficult resulting in missed endpoints.
A pre-specified analysis of less severe patients showed meaningful clinical results, and robust biomarker results across neuroinflammation, neuroprotection and neurodegeneration. This early patient population will be evaluated in the Phase 3b trial.
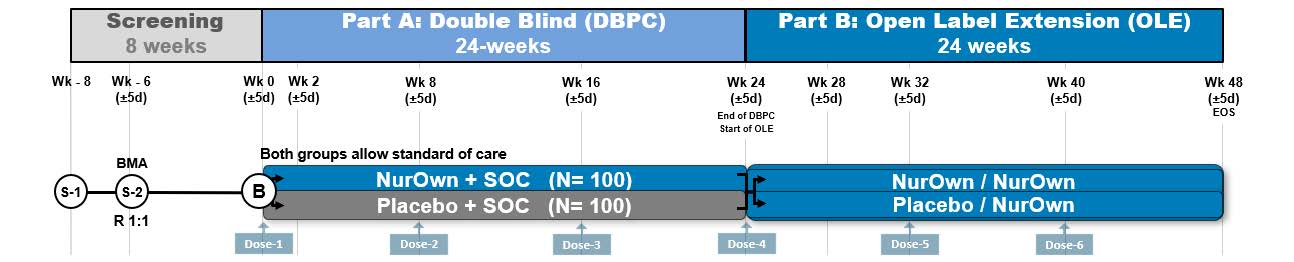
The planned Phase 3b target enrollment is approximately 200 patients in the United States. The study design is a two-part, multi-center, Phase 3b study to assess the efficacy and safety of NurOwn in participants with ALS who are earlier in their disease. Following screening, eligible patients will undergo bone marrow aspiration and enter a 24-week randomized, double-blind, placebo-controlled period (Part A), followed by a 24-week open-label extension period (Part B). Participants will be randomized 1:1 to the NurOwn and placebo groups. All participants can receive standard-of-care treatment. NurOwn or placebo will be administered by intrathecal injection every eight weeks.
The clinical trial protocol and statistical analysis plan for the upcoming Phase 3b trial of NurOwn have been granted a Special Protocol Assessment (SPA) by the Food and Drug Administration. This indicates that they are adequate for addressing objectives that support a future Biologics License Application (BLA) for ALS.
NurOwn Phase 3b Clinical Trial Design in ALS
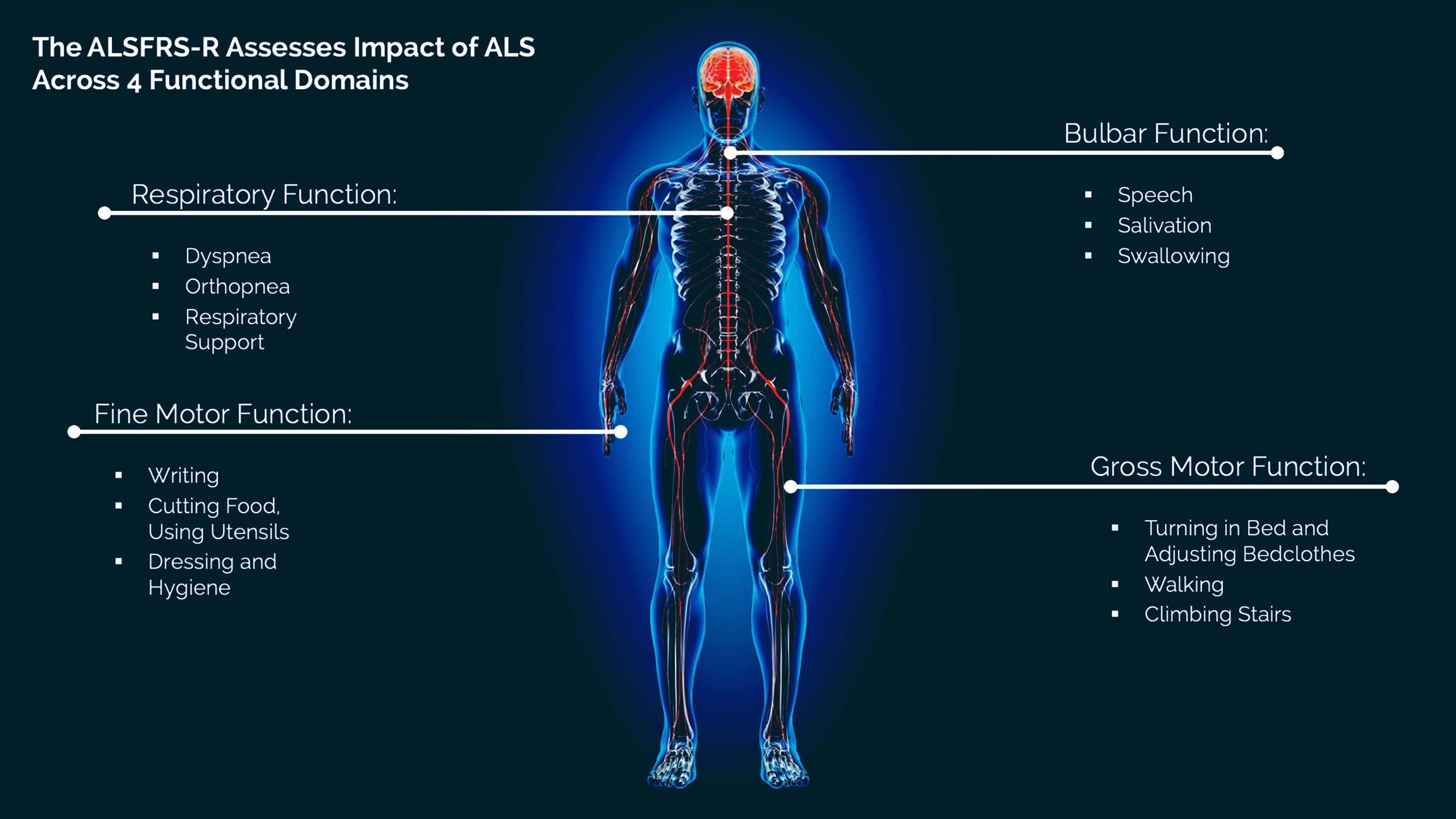
About the ALSFRS-R
The ALS Functional Rating Scale–Revised (ALSFRS-R) is a widely accepted measure of disease progression in ALS that is used in clinical trials.2,3 It is a validated questionnaire that measures physical function in performing activities of daily living (ADLs). Other measures of disease progression commonly used in clinical trials include assessment of muscle strength and respiratory function.
The ALSFRS-R measures 12 aspects of physical function across four functional domains (bulbar, fine motor, gross motor, and respiratory).
Source: Cedarbaum JM, et al. J Neural Sci. 1999;169:13-21.
About Progressive MS
Multiple Sclerosis (MS) is a chronic neuroinflammatory and neurodegenerative disorder that affects the brain and spinal cord.
There are multiple types of MS. The most common form of MS is relapsing-remitting MS (RRMS, 85% of MS patients), characterized by periods of relapse, with flare-ups or exacerbations of symptoms, and periods of remission. Progressive MS, includes both primary progressive MS (PPMS) and secondary progressive MS (SPMS), is characterized by a worsening of neurologic function and increase in disability over time.6 About 10% of people with MS are diagnosed with PPMS and about half of those diagnosed with RRMS will eventually transition to SPMS. Onset can occur at any age between 20-70. 7
There are no available therapies that halt the progression of established disability or result in functional improvement in progressive MS. Therapies addressing regeneration and repair may offer an innovative treatment option to address a currently unmet medical need. 8
Evidence from preclinical models of MS (EAE mouse model) suggests that MSC-NTF cells have the potential for immunomodulation, remyelination, and neuroprotection. 9-11
In preclinical studies, intracerebroventricular administration of MSC-NTF cells in EAE mice delayed symptom onset and improved survival. Read more.
Clinical Development
Program in Progressive MS
BrainStorm has completed a phase 2 open-label trial using repeat-administration of autologous MSC-NTF cells in progressive MS as defined and diagnosed by the recent 2017 revised McDonald Criteria (NCT03799718).
RECENT BRAINSTORM PUBLICATIONS AND PRESENTATIONS
Debamestrocel multimodal effects on biomarker pathways in amyotrophic lateral sclerosis are linked to clinical outcomes
Erratum issued to A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
Supplemental file with updated graphs from the Erratum – A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
A Randomized Placebo-Controlled Phase 3 Study of Mesenchymal stem cells induced to secrete high levels of neurotrophic factors in Amyotrophic Lateral Sclerosis
Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis
Therapeutic Benefits of MSC-NTF (NurOwn®) Exosomes in Acute Lung Injury Models
REFERENCES
1. Brown RH and Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162-172.
2. Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169(1-2):13-21
3. Leigh PN, Swash M, Iwasaki Y, et al. Amyotrophic lateral sclerosis: a consensus viewpoint on designing and implementing a clinical trial. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:84-98.
4. Kaufmann P, Levy G, Thompson JL, et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology. 2005;64:38-43.
5. Castrillo-Viguera C, Grasso DL, Simpson E, et al. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11:178-180.
6. Lublin FD, et al. Defining the clinical course of multiple sclerosis. Neurology. 2014;83:278-286.
7. Confavreux C and Vukusic C. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129:606-616.
8. Chataway J. Tackling progression in multiple sclerosis. Editorial. The Lancet Neurology June 2018.
9. Harris, VK, et al. Characterization of autologous mesenchymal stem cell-derived neural progenitors as a feasible source of stem cells for central nervous system applications in multiple sclerosis. Stem Cells Transl Med. 2012;1:536-547.
10. Martins LF, et al. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Scientific Reports. 2017;7:4153. DOI:10.1038/s41598-017-03592-1; 11.
11. Rivera FR and Aigner L. Adult mesenchymal stem cell therapy for myelin repair in multiple sclerosis. Biol Res. 2012;45:257-268.
